Have you ever wondered what makes a molecule behave the way it does? Why are some molecules more reactive than others, or why do certain chemical reactions happen at all? The answer lies in the intricate dance of electrons within a molecule, a dance that’s influenced by the presence of specific groups of atoms. These groups, known as electron withdrawing and donating groups, act like tiny molecular conductors, directing the flow of electrons and shaping the molecule’s reactivity. Understanding these groups is like unlocking a secret code, allowing you to predict and manipulate the behaviour of molecules.

Image: www.youtube.com
Today, we’ll delve into the fascinating world of electron withdrawing and donating groups. Get ready to explore the intricacies of chemical bonds, the influence of electronegativity, and how these groups play a crucial role in shaping the chemical world around us. We’ll unravel the fundamental concepts behind these groups and uncover their application in fields like organic chemistry and drug development.
The Two Sides of the Electron Flow: Electron Withdrawing and Donating Groups
Imagine a molecule as a bustling marketplace, with electrons constantly moving around, exchanging energy and information. Now, picture a group of atoms within this marketplace: electron withdrawing groups act like a group of merchants, attracting electrons towards themselves, creating a “pull” on the electron density within the molecule. On the other hand, electron donating groups are like generous patrons, pushing their electron density towards the rest of the molecule, making it more electron-rich.
To better understand how these groups work, let’s explore the core concepts that govern their behavior:
1. Electronegativity: The Tug of War for Electrons
Electronegativity is a fundamental concept that governs the ability of an atom to attract electrons towards itself. Atoms with higher electronegativity exert a stronger pull on shared electrons in a chemical bond, making them more likely to become negatively charged. This concept is key to understanding the behaviour of electron withdrawing and donating groups.
Electron withdrawing groups consist of atoms with high electronegativity, like halogens (fluorine, chlorine, bromine, iodine), oxygen, and nitrogen. These atoms, like greedy merchants, attract electrons towards themselves, pulling electron density away from the rest of the molecule. This creates a partial positive charge on the adjacent atoms, making the molecule more susceptible to attack by electron-rich species like nucleophiles.
Electron donating groups are made up of atoms with low electronegativity, like alkyl groups (carbon-hydrogen chains) or groups containing lone pairs of electrons, such as hydroxyl (-OH) or amino (-NH2) groups. These atoms are more likely to share their electrons, pushing electron density towards the rest of the molecule, creating a partial negative charge on the adjacent atoms. This makes the molecule more susceptible to attack by electron-deficient species like electrophiles.
2. Induction: The Ripple Effect of Electron Density
Imagine dropping a pebble into a still pond. The ripples that spread outward are analogous to the inductive effect in molecules. Electron withdrawing and donating groups induce changes in electron density throughout the entire molecule. These changes in electron density impact the reactivity of the molecule and influence how it interacts with other molecules.
Electron withdrawing groups create a “pull” on electron density, creating a “positive inductive effect.” This effect, like the ripples, spreads throughout the molecule, reducing the electron density in neighboring atoms and making the molecule more electrophilic.
Electron donating groups push electron density, creating a “negative inductive effect.” This effect increases the electron density in neighboring atoms, making the molecule more nucleophilic.
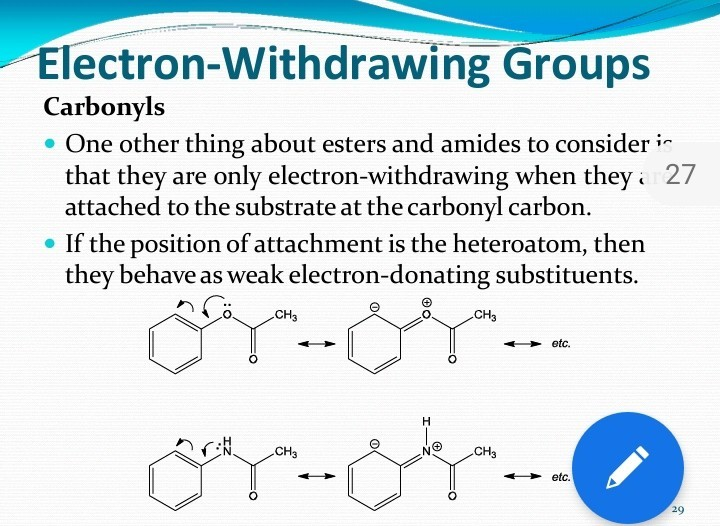
Image: notariaurbina.cl
3. Resonance: The Delocalization Dance of Electrons
Imagine electrons as tiny dancers constantly shifting and sharing their positions within a molecule. Resonance describes this movement of electrons, with the electrons delocalizing across multiple atoms within the molecule. This delocalization can be significantly influenced by the presence of electron withdrawing and donating groups.
Electron withdrawing groups can stabilize delocalized electrons by drawing them towards themselves, enhancing the resonance effect. This leads to increased stability and less reactivity.
Electron donating groups can further delocalize electrons by providing additional electron density, amplifying the resonance effect. This increased delocalization often leads to enhanced reactivity and more stable intermediates during chemical reactions.
The Impact of Electron Withdrawing and Donating Groups
These groups play a critical role in shaping the chemical world, influencing the properties and reactivity of molecules. Their impact extends to various fields:
1. Organic Chemistry: The Building Blocks of Life
Electron withdrawing and donating groups heavily influence the behavior of organic molecules, affecting their acid-base properties, reactivity towards various reagents, and the stability of intermediates during reactions. They play a crucial role in organic synthesis, allowing chemists to design and create complex molecules with desired properties.
2. Drug Development: Targeting Specific Molecules
In drug development, understanding the electronic properties of molecules is essential. By strategically incorporating electron withdrawing and donating groups, researchers can design molecules that target specific receptors or enzymes. This allows for the development of drugs with high affinity and selectivity, reducing side effects and maximizing therapeutic effectiveness.
3. Materials Science: Building New Materials
These groups are critical for tailoring the properties of materials, influencing their electrical conductivity, thermal stability, and mechanical properties. By strategically incorporating electron withdrawing and donating groups, scientists can design materials with specific applications in electronics, energy storage, and construction.
Expert Insights and Actionable Tips
“Understanding the concept of electron withdrawing and donating groups is essential for any chemist, whether you’re studying organic chemistry, bioorganic chemistry or designing new materials,” says Dr. Emily Carter, a renowned chemist and professor at Princeton University. “By incorporating these concepts into your thinking, you gain a powerful tool for predicting molecular behaviour and fine-tuning properties. It’s a game changer for anyone looking to understand the complex world of molecules.”
To leverage this knowledge:
-
Invest time in understanding the concepts thoroughly. Explore online resources, textbooks, and tutorials.
-
Practice applying the concepts, identifying the groups and predicting their effect on molecule properties.
-
Engage in discussions with peers and mentors to deepen your understanding.
List Of Electron Withdrawing Groups And Donating Groups
Conclusion
The world of molecules is a fascinating tapestry, woven with intricate relationships and delicate interactions. Electron withdrawing and donating groups act as the threads that guide this tapestry, shaping the properties and reactivity of molecules. By understanding these groups, we unlock a deeper understanding of the chemical world, allowing us to predict and control the behaviour of molecules. From designing new drugs to creating innovative materials, this knowledge opens up a world of possibilities. So delve into the world of electron withdrawing and donating groups, and begin exploring the exciting possibilities that await.






