Have you ever wondered how a rusty bike transforms from sleek metal to an orange, flaky mess? Or how a cake batter, seemingly simple, transforms into a fluffy delight in the oven? These changes, from the everyday to the extraordinary, are driven by chemical reactions – the fundamental processes that govern the universe around us.

Image: answermediabrandt.z19.web.core.windows.net
This worksheet delves into the exciting world of chemistry, specifically focusing on identifying various types of chemical reactions. It’s a must-have for any budding chemist, science enthusiast, or anyone curious about the fascinating transformations happening around us. Ready to unlock the mysteries of chemical reactions? Let’s begin!
Understanding the Fundamentals: A Glimpse into Chemical Reactions
At their core, chemical reactions involve the rearrangement of atoms and molecules, creating entirely new substances. Think of them as a dance of atoms, breaking old bonds and forming new ones, leading to a dramatic change in the composition of matter.
Visualize a simple example: burning wood. The wood, primarily composed of carbon, reacts with oxygen in the air. This reaction breaks apart the carbon and oxygen molecules, re-forming them into carbon dioxide and ash, a process releasing energy in the form of heat and light. This is just a glimpse into the fascinating world of chemical reactions.
Decoding the Types: Classifying Chemical Reactions
To make sense of the diverse world of chemical reactions, chemists have developed a system of classification. Just like a librarian organizing books by genre, we can categorize chemical reactions by their common features:
1. Synthesis Reactions (Combination Reactions)
Imagine two building blocks joining hands to create something bigger and more complex. This is the essence of synthesis reactions. Here, two or more reactants combine to form a single, more complex product.
Take the example of iron rusting. Iron (Fe) reacts with oxygen (O2) in the air to form iron oxide (Fe2O3), commonly known as rust. This combination reaction represents the formation of a new compound, iron oxide, from two simpler reactants.
General equation: A + B → AB
Example: 2Na + Cl2 → 2NaCl (Sodium reacts with chlorine to form sodium chloride)
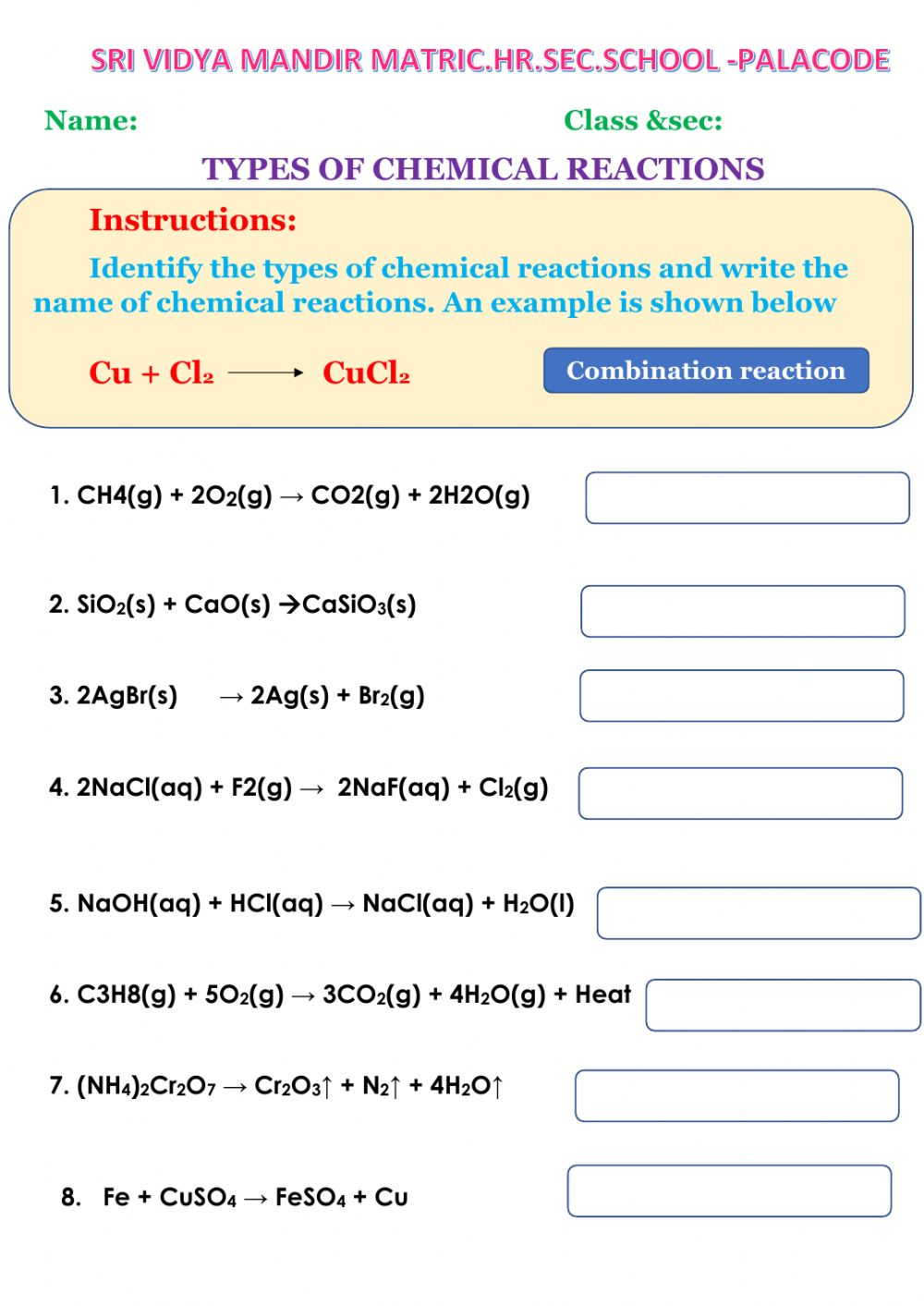
Image: printableschoolschulths.z19.web.core.windows.net
2. Decomposition Reactions
Now, picture a complex molecule breaking down into simpler units. This is decomposition at its finest! In these reactions, a single reactant breaks down into two or more products.
Imagine a chef carefully separating the ingredients of a cake batter. Similarly, decomposition reactions involve breaking down a compound into its constituent parts. The decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2) is a classic example.
General equation: AB → A + B
Example: CaCO3 → CaO + CO2 (Calcium carbonate decomposes into calcium oxide and carbon dioxide)
3. Single Displacement Reactions
Think about a daring adventurer replacing another in a quest. This is analogous to single displacement reactions, where a more reactive element displaces another element from a compound.
Imagine a more reactive metal, zinc (Zn), being added to a solution of copper sulfate (CuSO4). The zinc, being more reactive, displaces the copper (Cu) from the compound, resulting in zinc sulfate (ZnSO4) and elemental copper.
General equation: A + BC → AC + B
Example: Zn + CuSO4 → ZnSO4 + Cu (Zinc displaces copper from copper sulfate)
4. Double Displacement Reactions
Imagine two partners swapping roles in a dance! Double displacement reactions involve the exchange of ions between two reactants, resulting in the formation of two new compounds.
Consider the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl). The silver ions (Ag+) from silver nitrate combine with chloride ions (Cl-) from sodium chloride to form a white precipitate, silver chloride (AgCl). Meanwhile, the sodium ions (Na+) from sodium chloride combine with the nitrate ions (NO3-) from silver nitrate to form sodium nitrate (NaNO3).
General equation: AB + CD → AD + CB
Example: AgNO3 + NaCl → AgCl + NaNO3 (Silver nitrate reacts with sodium chloride to form silver chloride and sodium nitrate)
5. Combustion Reactions
Imagine a bonfire radiating warmth and light, consuming fuel in the process. Combustion reactions, involving the rapid reaction between a substance with an oxidant, usually oxygen, produce heat and light.
The burning of fuels, like wood, natural gas, and gasoline, are excellent examples of combustion reactions. These reactions are vital for producing energy for various applications, from powering vehicles to generating electricity.
General equation: Fuel + Oxidant → Products (usually oxides) + Energy
Example: CH4 + 2O2 → CO2 + 2H2O (Combustion of methane, natural gas)
6. Neutralization Reactions
Imagine a battle between acidity and alkalinity, resulting in a peaceful truce. Neutralization reactions involve the reaction between an acid and a base, producing salt and water.
Consider the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH). The hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base, forming water (H2O). The remaining ions, sodium (Na+) and chloride (Cl-), combine to form sodium chloride (NaCl), a salt.
General equation: Acid + Base → Salt + Water
Example: HCl + NaOH → NaCl + H2O (Hydrochloric acid reacts with sodium hydroxide)
Mastering the Worksheet: Tips for Success
Now that we have explored the different types of chemical reactions, let’s equip you with tips for tackling your worksheet confidently:
-
Understand the Definitions: Revisit the definitions of each reaction type. Understanding the core concepts will make identifying reactions a breeze.
-
Focus on the Products: Pay close attention to the products formed in a reaction. Examining the products frequently reveals the type of reaction taking place. For example, if water is formed, it often indicates a neutralization reaction.
-
Practice, Practice, Practice: Like any skill, identifying chemical reactions requires practice. Work through the worksheet diligently, using the definitions and examples discussed earlier as a reference.
-
Don’t Be Afraid to Ask for Help: If you encounter a challenging question, don’t hesitate to reach out for guidance. Your teacher or a classmate’s insight can provide valuable assistance for understanding those difficult concepts.
Chemical Reactions Worksheet #1 – Identifying Reaction Types
Conclusion: A World of Reactions Awaits
By mastering the fundamentals of identifying chemical reactions, you unlock a world of scientific inquiry and understanding. These reactions shape our world, from the rust on your bicycle to the energy that powers our homes. Now, equipped with the knowledge and tips, embark on your worksheet journey, embrace the challenge, and discover the fascinating dance of atoms that shapes our existence!






