The world of chemistry is full of fascinating reactions, but few are as elegant and precisely controlled as titrations. As a student first learning about titrations, I vividly recall the thrill of watching the color of the solution change, signaling the endpoint of the reaction. The process was a perfect blend of theory and practical application, and the results felt like a tangible representation of the unseen world of molecules at work. This blog post explores the crucial role of a titration report sheet and how it helps us record and analyze data in these experiments.
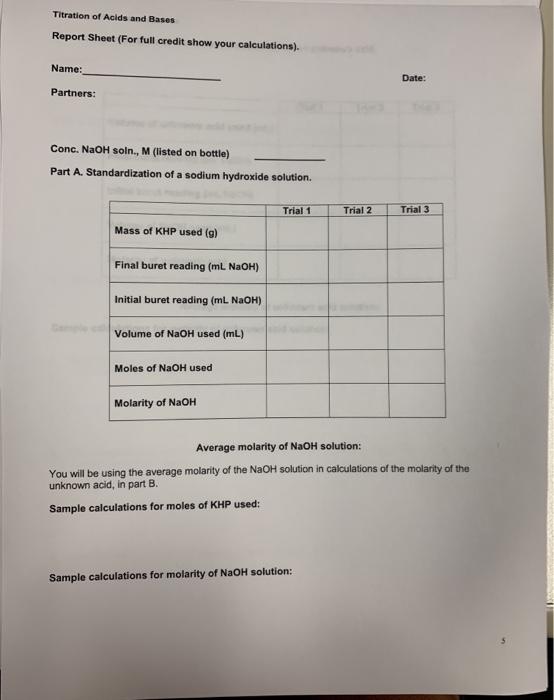
Image: www.chegg.com
Titration is a fundamental analytical technique in chemistry, offering a precise method to determine the unknown concentration of a solution. But it’s not just about the process itself; it’s about capturing the data meticulously to draw meaningful conclusions. That’s where the titration report sheet comes into play – it’s your trusted companion in the journey to mastering this technique.
The Importance of a Titration Report Sheet
A titration report sheet is more than just a blank sheet of paper; it’s a roadmap for your experiment. It ensures that you capture all the necessary information in a standardized and organized manner, facilitating accurate analysis and clear communication of your findings.
Imagine a detective investigating a crime scene. Just like the detective carefully examines every piece of evidence, you need to meticulously record every detail of your titration experiment. The report sheet acts as your evidence log, providing a comprehensive record of your experiment’s progress.
Understanding the Titration Process
What is Titration?
Titration is a quantitative chemical analysis technique used to determine the concentration of a known analyte (the solution with unknown concentration) by reacting it with a solution of known concentration, called the titrant. The titrant is added gradually to the analyte until the reaction is complete, evidenced by a color change, typically signalled by a chemical indicator. This point, known as the equivalence point, signifies that the moles of titrant added are equal to the moles of analyte present.
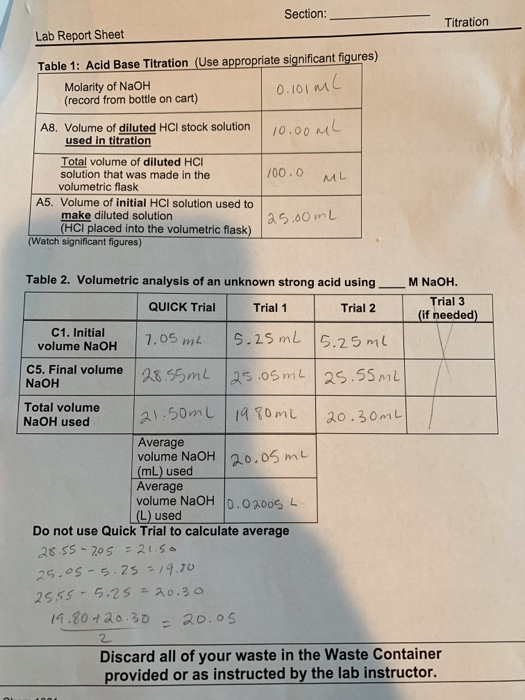
Image: www.chegg.com
Titration of Acids and Bases
In the case of acid-base titrations, the titrant is either a strong acid or a strong base, and the analyte is a solution of unknown concentration of either an acid or a base. The reaction is a neutralization reaction, where the acid and base react to form salt and water. For example, a strong acid like hydrochloric acid (HCl) can be used to titrate a solution of sodium hydroxide (NaOH), a strong base.
Types of Titration
Acid-base titrations, where we neutralize an acid with a base or vice versa, are not the only kind. There are also:
- Redox Titration: These involve the transfer of electrons between the titrant and analyte, like oxidation-reduction reactions.
- Precipitation Titration: These titrations form an insoluble precipitate during the reaction, helping to identify the endpoint.
- Complexometric Titration: In these titrations, a complex is formed between the analyte and the titrant, which can be used to determine the concentration of the analyte.
Essential Components of a Titration Report Sheet
A complete titration report sheet typically includes the following key sections:
1. Experiment Information:
- Title of the Experiment
- Date of Experiment
- Names of Students Involved
2. Materials and Equipment:
- Analyte solution (name and concentration, if known)
- Titrant solution (name and concentration)
- Burets, pipettes, beakers, flasks
- Indicator used (name and color change)
3. Procedure:
- A detailed step-by-step description of the titration procedure followed
- Mention any modifications made to the standard procedure
4. Observations and Data:
- Initial buret reading (in mL)
- Final buret reading (in mL)
- Volume of titrant used (calculated from initial and final readings)
- Number of trials conducted
- Color changes observed at the endpoint
- Any unusual observations or challenges faced during the titration
5. Calculations:
- Moles of titrant used (calculated using the volume and concentration of the titrant)
- Moles of analyte (calculated using the mole ratio from the balanced chemical equation)
- Concentration of the analyte (calculated using moles of analyte and the known volume of the analyte solution)
- Any other relevant calculations based on the experimental data
6. Results and Analysis:
- Summarize the calculated concentration of the analyte from each trial
- Calculate the average concentration of the analyte (if multiple trials were performed)
- Discuss the accuracy and precision of the results
- Analyze potential sources of error in the experiment
7. Conclusion:
- State the final determination of the analyte concentration
- Discuss the significance of the findings and whether the results were consistent with expectations
- Mention any potential limitations of the experiment
Tips and Expert Advice
Data Recording: The Key to Accuracy
Precise data recording is crucial for the success of any titration experiment. Here are some tips to ensure accuracy:
- Read the buret carefully: Make sure your eyes are level with the meniscus of the solution (the curved surface at the top) when taking readings. A single drop can significantly affect your results.
- Use the correct units: Always double-check that you’re using the correct units for volume, concentration, and other measurements. Using milliliters instead of liters can lead to significant errors in calculations.
- Record observations immediately: Don’t rely on memory; write down every observation, like the color change at the endpoint, as soon as you see it. It’s easy to forget crucial details later on.
Handling the Titrant: A Gentle Touch
The titrant, the solution you’re adding dropwise to the analyte, is the key to getting the correct endpoint. Improper handling of the titrant can easily lead to unusable results. Here’s how to avoid common mistakes:
- Slow and steady wins the race: Titrate slowly, especially near the endpoint. A sudden burst of titrant can overshoot the endpoint, making it difficult to determine the correct volume.
- Keep the buret clean: Ensure the buret is clean and free of any contaminants or residues that might interfere with the reaction or affect the accuracy of the readings.
- Don’t shake the buret: Shaking the buret can create air bubbles inside, which will affect the accurate delivery of the titrant. Always gently swirl the titration flask to ensure proper mixing.
Frequently Asked Questions
Q: What are the main sources of error in a titration experiment?
A: Common sources of error in titrations include:
- Improper buret reading: An inaccurate reading at the beginning or end of the titration can affect the volume of titrant used.
- Air bubbles in the buret: Air bubbles trapped in the buret will affect the volume of titrant delivered.
- Improper mixing: Insufficient mixing of the solution during the titration can lead to an inaccurate endpoint.
- Using the wrong indicator: The indicator should be carefully chosen to provide a sharp color change at the specific pH of the equivalence point. The wrong indicator can lead to an inaccurate endpoint.
- Contaminated equipment: Impurities in the buret, flasks, or other equipment can interfere with the titration reaction.
Q: How can I improve the accuracy of my titration results?
A: You can enhance the accuracy of your results by:
- Repeating the titration multiple times: This helps to identify and eliminate any errors or outliers.
- Using a standardized titrant: Ensure that the titrant solution is accurately prepared and standardized to ensure its concentration is known with high precision.
- Calibrating equipment: Regular calibration of the buret, pipettes, and other equipment is essential for accurate measurements.
- Thorough rinsing: Always rinse the buret, pipettes, and flasks with distilled water before and after using them to prevent cross-contamination.
Q: Can I use a different indicator in a titration experiment?
A: The choice of indicator depends on the specific titration you are performing. You need an indicator that changes color at the pH range closest to the equivalence point of the reaction. If you use a different indicator than the one recommended, you may not get an accurate endpoint, and your results might be unreliable.
Titration Of Acids And Bases Report Sheet
Conclusion:
In the world of chemistry, the accuracy of your experiments depends on your ability to carefully record and analyze data. A titration report sheet is your essential tool to document your titration experiments and achieve reliable results. From meticulously observed data points to meticulous calculations, each section of the report sheet plays a crucial role in understanding the concentration of the analyte and validating your findings.
Are you interested in learning more about titration techniques or have specific questions about the report sheet? Feel free to share your thoughts and questions in the comments below. We’re here to help you explore the fascinating world of chemistry!






