Have you ever wondered how seemingly simple molecules can be transformed into entirely different compounds, possessing entirely new properties? This fascinating process is the core of organic chemistry, and one of its most intriguing examples lies in the conversion of 1-butanol to 1-bromobutane. This seemingly simple transformation involves a series of intricate steps, each revealing the fundamental principles that govern chemical reactions.
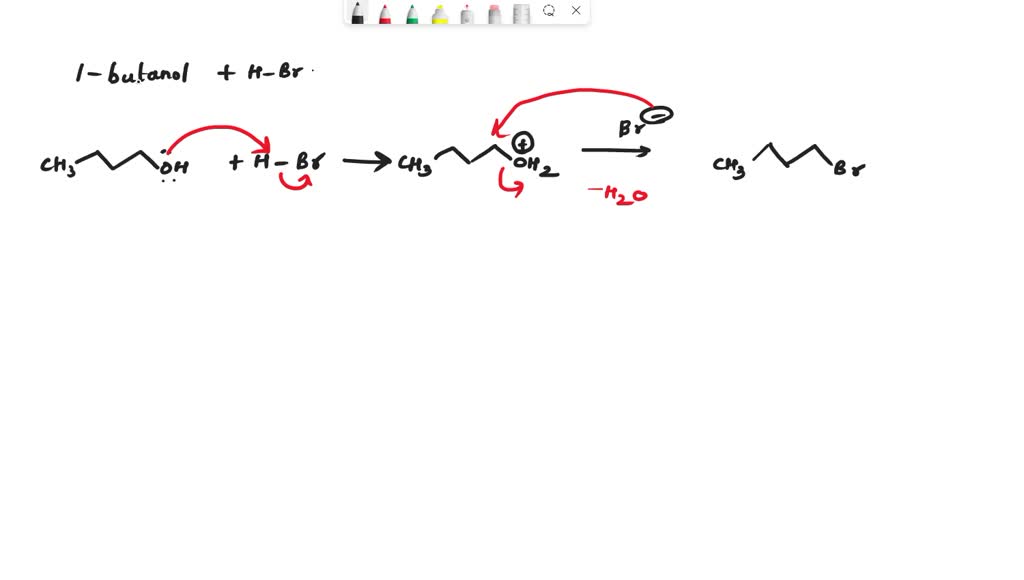
Image: www.numerade.com
Understanding the mechanism through which 1-butanol is transformed into 1-bromobutane is not only crucial for comprehending the basics of organic chemistry but also relevant to various fields, including pharmaceutical synthesis, materials science, and the development of new agrochemicals. This journey into the molecular world will shed light on the intricacies of reactions, highlighting the power and elegance of organic chemistry.
Unveiling the Starting Point: 1-Butanol
Our starting point, 1-butanol, is a simple alcohol with a relatively straightforward structure. It comprises a four-carbon chain with a hydroxyl (-OH) group attached to the first carbon. This hydroxyl group is crucial for the reaction’s success, as it acts as the site for the transformation. 1-Butanol finds extensive use as a solvent and intermediate in the production of various chemicals, ranging from plastics to fragrances.
The Transformation: From Alcohol to Alkyl Halide
The conversion of 1-butanol to 1-bromobutane involves replacing the hydroxyl group with a bromine atom. This seemingly simple substitution is achieved through a complex series of steps, each with its own unique mechanism. This transformation is a classic example of a nucleophilic substitution reaction, a central concept in organic chemistry.
The Key Player: The Nucleophile
The primary agent responsible for this transformation is the nucleophile, in this case, bromide ion (Br–). Nucleophiles are chemical entities that are attracted to positively charged centers, seeking to form new bonds. In our reaction, the bromide ion acts as a nucleophile, attracted to the positively charged carbon atom adjacent to the hydroxyl group in 1-butanol
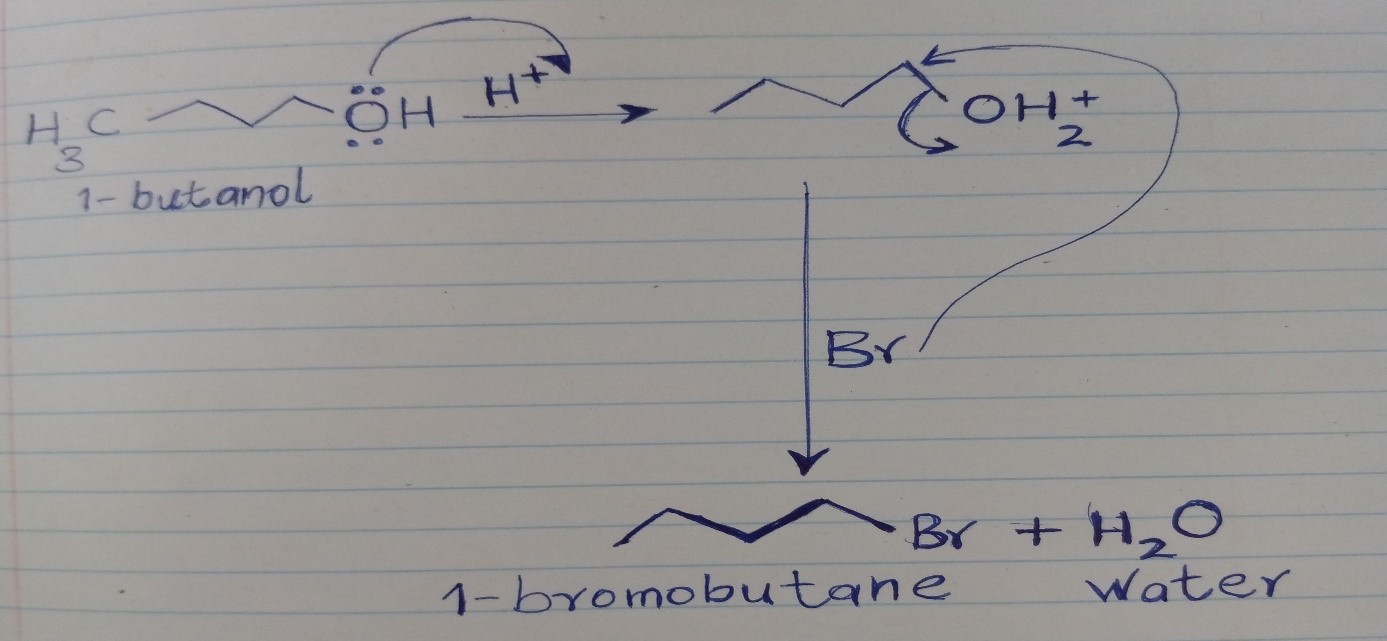
Image: peachyessay.com
The Catalyst: Setting the Stage for Success
The transformation is facilitated by the presence of a catalyst, commonly a strong acid like hydrobromic acid (HBr). The acid plays a crucial role in activating the hydroxyl group for substitution. By protonating the oxygen atom, the acid makes the hydroxyl group a better leaving group, meaning it is more easily replaced by the bromide ion.
The Mechanism: A Step-by-Step Breakdown
The reaction proceeds through a series of steps, each carefully orchestrated to yield the desired product.
Step 1: Protonation of the Hydroxyl Group
The reaction begins with the protonation of the hydroxyl group in 1-butanol by the acid catalyst. This protonation transforms the hydroxyl group into a better leaving group, preparing it for replacement by the bromide ion.
Step 2: Formation of a Carbocation Intermediate
Once the hydroxyl group is protonated, the oxygen atom, now positively charged, departs from the molecule, leading to the formation of a carbocation intermediate. The carbocation, a species with a positively charged carbon atom, is highly reactive and readily seeks to regain its electron stability.
Step 3: Nucleophilic Attack
The bromide ion, acting as the nucleophile, attacks the positively charged carbon atom in the carbocation intermediate. This attack forms a new bond between the carbon and the bromine atom, resulting in the desired product, 1-bromobutane.
Understanding the Importance of 1-Bromobutane: Applications in the Real World
1-Bromobutane, produced through this specific reaction, plays a vital role in various industrial sectors.
- Pharmaceutical Synthesis: 1-Bromobutane serves as an essential building block in the synthesis of pharmaceuticals, contributing to the development of new medicines.
- Materials Science: Its properties make it a valuable reagent in the fabrication of specialized polymers and materials with desired characteristics.
- Agrochemicals: 1-Bromobutane is an essential component in the synthesis of pesticides, helping to control pests and enhance crop yields.
Beyond the Reaction: Considerations for Efficiency and Safety
While the reaction provides an efficient method for producing 1-bromobutane, it’s important to consider safety precautions and environmental impact.
- Safety: 1-Bromobutane is a highly flammable and toxic substance. Handling it requires utmost caution and adherence to safety protocols.
- Environmental Impact: The use of strong acids and the potential presence of byproducts necessitate responsible disposal practices to minimize environmental impact.
1 Butanol To 1 Bromobutane Mechanism
Conclusion: A Journey into the Intricacies of Chemistry
The conversion of 1-butanol to 1-bromobutane reveals the intricacies and elegance of organic chemistry. This seemingly simple transformation involves a carefully orchestrated series of steps, each governed by fundamental chemical principles. Understanding these principles allows us to manipulate molecules and create new compounds with diverse properties. The importance of 1-bromobutane in various industries highlights the real-world significance of this reaction, serving as a bridge between theoretical chemistry and practical applications. Through this journey into the world of chemical transformations, we gain an appreciation for the power and precision that governs the molecular world.






